Review Questions, Final Exam, 28 Jan. 2010
Equilibrium - see http://faculty.uncfsu.edu/dautrey/CHEM%20160/Chapter%2015%20sample%20problems.pdf
These are the questions that were given in class on Wed. 26 Jan.
[Answers for questins 1 to 12 are given after question 12]
1. The equation representing the predominant reaction of sodium ethanoate, NaCH3COO, with water is
answer: d) CH3COO- + H20 <--> CH3COOH + OH-
2. What is the pH of the solution of when a small amount of 0.040 mol NaOH(s) is added to
1.00 L of 0.050 M HCl?
a) 2.00 b) 1.40 c) 1.30 d) 7.00 e) 8.50
3. Which of the following represents a redox reaction?
answer: a) SiCl4 + 2Mg --> Si + 2MgCl2
4. In a nickel-cadmium barttery: NiO2 (s) + Cd(s) + 2H2) --> Ni(OH)2 + Cd(OH)2 . Which of the following occurs at the anode as the reaction proceeds?
a) NiO2 gains 2e- and forms Ni(OH)2(s)
b) NiO2 loses 2e- and forms Ni(OH)2(s)
C) Cd gains 2e- and forms Cd(OH)2(s)
d) Cd loses 2e- and forms Cd(OH)2(s)
5. Consider the following reaction:
2NO(g)+ O₂(g) à NO₂ (g) ∆H = -114 kJ
How could the rate of this reaction be increased?
A. Reduce the pressure.
B. Increase the volume.
C. Remove some NO2(g) .
D. Increase the temperature.
6. (picture can not copy here ). The answer is d.
7. An activated complex can be described as
A. a particle of maximum KE and minimum PE.
B. a stable particle found in a reaction mechanism.
C. an unstable particle that is neither reactant nor product.
D. a particle which is first used then regenerated in a reaction mechanism.
8. Which of the following could result in an increase in reaction rate?
A. an increase in the activation energy
B. an increase in the reaction enthalpy
C. an increase in the frequency of collisions
D. an increase in the potential energy of the activated complex
9. Consider the following equilibrium: CO₂(g) + 2 H₂(g) à CH₃OH(g) ∆H = - 91 kJ +
Which of the factors below would increase the concentration of CH₃OH at equilibrium?
A. an addition of CO₂
B. an increase in the volume
C. a decrease in the pressure
D. an increase in the temperature
10. What does a buffer solution usually contain?
A. a strong acid and its conjugate acid
B. a strong acid and its conjugate base
C. a weak acid and its conjugate acid
D. a weak acid and its conjugate base
11. Acidified potassium permanganate (KMnO4) solution is often used in redox
titrations. Permanganate reacts with Sn2+ as follows: MnO₄¯ + Sn²⁺ --> Mn²⁺ + Sn⁴⁺
When the equation is balanced, the number of Sn²⁺ ions is:
a) 1 b) 2 c) 3 d) 4 e) 5
12. What is the reducing agent in the reaction in question 11?
a) Mn²⁺ b) Sn⁴⁺ c) Sn²⁺ d) MnO₄⁻
Answers for 1 - 12:
1. d, 2. a, 3. a, 4. d, 5. d, 6. d,
7. c, 8. c, 9. a, 10. d, 11. e, 12. c
13. Balance this redox reaction H₂S + CrO₄²⁻ --> S₈ + Cr³⁺ in acidic solution
80 H+ + 24 H2S + 16 (CrO4)2- --> 3 S8 +16 Cr3+ + 64 H2O
14. Hydrogen peroxide decomposes according to the following thermochemical reaction:
H2O2(l) → H2O(l) + 1/2 O2(g); ΔH = -98.2 kJ Molar mass of H2O2 is 34.0
Calculate the change in enthalpy, ΔH, when 1.00 g of hydrogen peroxide decomposes.
-2.89 kJ
15. How much energy in kJ must be removed from 225 g of water in order to lower its temperature from 25.0 C to 10.0 C? 14.1 kJ
16. When 13.4g of ammonium chloride is mixed with 2.00x102 g of water it dissociates into ions and causes the temperature of the solution to drop from 20.0oC to 15.3oC. Determine the molar enthalpy of solution for ammonium chloride. (Ans: +15.7kJ/mol)
17. The enthalpy of combustion for H2, C(graphite) and CH4 given below. Calculate the standard enthalpy of formation ∆Hf for CH4.
(1) H2(g) + 0.5 O2(g) -> H2O(l) ∆H°f = -285.8 kJ/mol
(2) C(graphite) + O2(g) -> CO2(g) ∆H°f = -293.5 kJ/mol
(3) CH4(g) + 2O2(g) -> CO2(g) + 2H2O(l) -890.4 kJ/mol
18 . From the following data,
CH4 + 2O2 -> CO2 + 2H2O ∆H = -890 kJ/mol
H2O(l) -> H2O(g) ∆H = 44 kJ/mol at 298 K
Calculate the enthalpy of the reaction
CH4 + 2 O2(g) -> CO2(g) + 2 H2O(g) ∆H = ? - 802 kJ/mol
19. The enthalpies of formation of H2O, H2S, and SO2 are -285.8, -20.6 and -296.8 kJ/mol respectively. Calculate the standard enthalpy of change for the reaction, 2 H2S + SO2 -> 3 S + 2 H2O
All reactants and products are at their standard states.
-233.6 kJ (Use Hess's Law)
20. What is the enthalpy change when 12.8g H2(g) reacts with excess Cl2(g) to form HCl(g)? H2(g) + Cl2(g) à 2HCl(g) ∆H = -184.6kJ -1.17 X 10 to the 3 kJ
21. A burner on an electric stove has a heat capacity of 345J/K. What is the value of q, in kilojoules, as the burner cools from a temperature of 467°C to a room temperature of 23°C? -153 kJ
22. What mass of water, in kilograms, can be heated from 5.5°C to 55.0°C by 9.09 x 10 ⁱ⁰ J of heat? 4.39 X 10 to the 5 kg
23. A 454g block of lead is at an initial temperature of 22.5°C. What will be the temperature of the lead after it absorbs 4.22kJ of heat from its surroundings? 95.1 C
24. Calculate the value of the equilibrium constant, Kc , for the system shown, if 0.1908 moles of CO2, 0.0908 moles of H2, 0.0092 moles of CO, and 0.0092 moles of H2O vapor were present in a 2.00 L reaction vessel were present at equilibrium
Co2 + H2 <--> CO + H2O 4.9 x 10 to the -3 = K
25. Initially, a mixture of 0.100 M NO, 0.050 M H2, 0.100 M H2 O was allowed to reach equilibrium (initially there was no N2 ). At equilibrium the concentration of NO was found to be 0.062 M. Determine the value of the equilibrium constant, Kc , for the reaction: 2 NO + 2H2 <--> N2 + 2 H2O K = 650
26. A container is charged with 3.00 atm of dinitrogen tetroxide gas and 2.00 atm of nitrogen dioxide gas at 25oC and allowed to reach equilibrium. It was found that the pressure of the nitrogen dioxide decreased by 0.952 atm. Estimate the value of Kp for this system: Kp is similar to Kc and the calculations are done the same way except that you use pressure in atmospheres instead of concentration. N2O4 <--> 2 NO2
K = 0.3160
27. Phosgene (COCl2) is a poisonous gas that dissociates at high temperature into two other poisonous gases, carbon monoxide and chlorine. The equilibrium constant Kp = 0.0041 at 600°K. Find the equilibrium composition of the system after 0.124 atm of COCl2 is allowed to reach equilibrium at this temperature. Assume that x is very small.
0.104 atm for COCl2 and 0.0206 atm for CO and Cl2
28. Calculate the concentrations for each species present in a 0.1000 M aqueous solution of nitrous acid (Ka = 6.0 x 10-4). HNO2(aq) + H2O(l) H3O+(aq) + NO2-(aq)
[HNO2] = 0.100-x = 0.0926M
29. What is the hydronium ion concentration in a water solution that is 0.050 M NaOH?
2 x 10 -13 M
30. What is the hydroxide ion concentration in a water solution that is 4.0 x 10-5 M H3O+?
2.5 x 10 -10 M
31. What is the pKa of acetic acid, if Ka for acetic acid is 1.78 x 10-5?
4.75
32. Calculate the value of the ionization constant for the ammonium ion, Ka, if the pKa is 9.74.
1.82 x 10 -10
33. What is the pKb for methyl amine, if the value of Kb for methyl amine is 4.4 x 10-4?
3.36
34. Calculate the value of the ionization constant, Kb, for aniline if the pKb is 9.38.
4.17 x 10 -10
35. The Ka for acetic acid is 1.7 x 10-5. What is the value of Kb for the acetate ion?
5.9 x 10 -10
36. The Kb for aniline is 3.8 x 10-10. What is the value of Ka for the anilonium ion?
2.6 x 10 -5
37. What is the pH of a 0.400 M KBr solution?
neutral, pH = 7
38. To determine the initial concentration of a reactant A, we need to know: e
a) The final concentration of A
b) The length of time, t, the reaction ran to reach the final concentration.
c) The order of the reaction or enough information to determine it.
d) The rate constant, k, for the reaction or enough information to determine it.
e) All of the above.
39. Calculate the solubility product constant (Ksp) for lead(II) chloride, if 50.0 mL of a saturated solution of lead(II) chloride was found to contain 0.2207 g of lead(II) chloride dissolved in it. PbCl2(s) --> Pb2+(aq) + 2 Cl-(aq)
1.61 x 10 -5
40. Estimate the solubility of Ag2CrO4 in pure water if the solubility product constant for silver chromate is 1.1 x 10-12.
Ag2CrO4(s) --> 2 Ag+(aq) + CrO42-(aq)
[CrO4] = 6.5 x 10 -5 M
41. Estimate the solubility of barium sulfate in a 0.020 M sodium sulfate solution. The solubility product constant for barium sulfate is 1.1 x 10-10. BaSO4(s) --> Ba2+(aq) + SO42-(aq) [note: common ions!]
5.5 x 10 -9 M is x in the ICE table
42. 25.0 mL of 0.0020 M potassium chromate are mixed with 75.0 mL of 0.000125 M lead(II) nitrate. Will a precipitate of lead(II) chromate form. Ksp of lead(II) chromate is 1.8 x 10-14. yes we will look at this in class tomorrow (Thursday)
Review Questions, Redox Quiz Wed. 26 Jan 2010
see homework questions from the past few days as well
1. In a redox reaction, a reducing agent becomes oxidized. True or False?
2. An atom or ion is oxidized if it's oxidation number increases in a redox reaction. True or false?
3. Oxidation occurs in the anode half cell in an electrochemical cell. True or false?
4. An electrochemical cell has two solid electrodes. True or false?
5. If an ion loses electrons in a redox reaction, it has been oxidized. True or false?
6. Balance this redox reaction: H₂S + CrO₄²⁻ --> S₈ + Cr³⁺ in acidic solution.
7. In the reaction in question 6, what is the reducing agent?
8. Write a chemical equation showing the oxidation of an iron ion.
9. What is the purpose of the salt bridge in a galvanic cell?
10. Describe what happens to electrons in an electrochemical cell. Draw pictures if needed.
--------------------------------------------------------------
Review Questions, Chemistry Quiz, Thursday 20 Jan., 2010
I'll be adding questions until about 8:30 p.m. this evening (19 Jan)
1. Strong acids conduct electricity ____________________ than weak acids.
2. What is the definition of a Lewis acid?
3. Naturally occurring acids are _____________ acids (strong or weak?)
4. If you have a 1.2 M solution of NaOH, what is the [OH-]?
5. What is the difference between a monoprotic acid and a diprotic acid?
6. Name 2 strong acids.
7. What is a neutralization reaction?
8. What is a hydronium ion?
9. F- is a weak base. What is its conjugate acid?
10. HClO4 is a strong acid. What is its conjugate base? Is its conjugate base a weak base or a strong base?
11. If the Ka of an acid HA is 3 x 10-5, what is the Kb of the ion A-? What is the pKa of HA?
12. In the reaction, H2PO4- + HS- --> H2S + HPO42- which ions are the acid and the base?
13. In the reaction B + H20 --> BH + OH- where B is a base, what is the conjugate acid of B?
14. What is the difference between a cation and an anion?
15. A strong acid reacts with a weak base and a salt is formed. Will it probably be an acidic salt or a basic salt?
16. What does the word alkaline mean?
17. Write a balanced chemical equation for the reaction of Ba(OH)2 in water.
18. The metal oxide, MgO (solid) is put in a beaker of water. A small amount of MgO dissolves in the water. Is the aqueous solution acidic or basic?
19. What is Tris?
20. Why is HCO3- called amphoteric?
21. What are 3 differences between electrophiles and nucleophiles?
22. What is a coordinate covalent bond?
23. If a a substance R-COOH has a pKa of 5 and it is in a solution of pH 7, is that substance likely to be charged or neutral?
24. Acid A has a pKa of 1 and acid B has a pKa of 4. Which acid is stronger?
25. If HA has a pH of 4.5, what is its pOH?
26. Buffers are often made with a weak base and its ___________________ .
27. Write the chemical equation for any hydrolysis reaction.
28. Write the chemical equation for any neutralization reaction.
29, If a solution 0.5 M of acid, HA, is 25% ionized at equilibrium, what is the [HA] at equilibrium?
30. What is the pH of a 0.15 M solution of methylamine CH3NH2 if the Kb = 4.4 x 10-4 ?
31. What is the conjugate acid of CH3NH2?
32. What is the Kw and pKw of water?
33. If you wanted to make a 1L solution of pH = 2, how much HCL should you add to 1L of water? Ignore the volume change after adding HCl.
34. pH is dependent on concentration, true or false?
35. What is an electrolyte?
Part B
1. Which of the following 0.1 M solutions will have the greatest electrical conductivity?
a) HNO2
b) H2SO3
c) H3PO4
d) C6H5OH
2. Consider the following equilibrium
2H2O(l ) ⇄ H3O+ (aq) + OH- (aq)
A small amount of HCl is added to water and equilibrium is reestablished. When comparing the new equilibrium with the original equilibrium,
a) [H3O+] and pH both decreased
b) [H3O+] and pH both increased
c) [H3O+] increased and pH decreased
d) [H3O+] decreased and pH increased
3. A Brönsted-Lowry base is defined as a chemical species that
a) accepts protons
b) neutralizes acids
c) donates electrons
d) produces hydroxide ions in solution
4. In a solution with a [OH-] of 1.5 x 10-4 M, the [H3O+] is
a) 6.7 x 10-11 M
b) 1.0 x 10-7 M
c) 1.5 x 10-4 M
d) 1.2 x 10-2 M
5. The pH of pure water is 6.52 at 60°C. The [OH-] is
a) 3.3 x 10-8 M
b) 1.0 x 10-7 M
c) 3.0 x 10-7 M
d) 8.1 x 10-1 M
6. Which of the following oxides, when dissolved in water, will produce the most basic solution?
a) BaO
b) CO2
c) SO2
d) ClO
7. Which of the following could act as a Brönsted-Lowry acid, but not as a Brönsted-Lowry base?
a) HC1O4(aq)
b) H2O(l)
c) NH3(aq)
d) HCO3(aq)
8. The strength of an acid depends upon its
a) E°
b) pH
c) concentration
d) degree of ionization
9. Consider the following equilibrium:
HX- + HZ- ⇄ H2X + Z 2-
The reactants are favoured. The strongest acid is
a) Z 2-
b) HZ
c) HX
d) H2X
10. The pH of a 0.025 M NaOH solution is
a) 0.94
b) 1.60
c) 12.40
d) 13.06
Part C
1. Nicotinic acid, HC6H4NO2, is a weak acid found in vitamin B.
Calculate the pH of 0.100 M HC6H4NO2 (Ka = 1.4 x 10-5).
2. What is the [H3O+] in a solution formed by adding 60.0 mL of water to 40.0 mL of 0.040 M KOH?
3. Calculate the pH in 100.0 mL of 0.400 M CH3COOH (Ka = 1.8 x 10-5 is a weak acid)
4. The salt NaCN dissolves in water and forms a slightly basic solution. Write the dissociation equation for
NaCN in water. (the chemical equations for what happens when NaCN dissolves in water)
--------------------------------------------------------------------------------------------------------------
for Tuesday, 14 Dec
Don`t forget to review WHMIS symbols for hazardous materials! Page 790 & 791
Review questions, pages 282 and 283 of text
Self-quiz, pages 286 - 289 of the text
Part 1
1. Draw an example of a metallic bonding.
2. Draw an example of an ionic crystal.
3. Draw the shape and arrangement of the sp3 hybrid orbitals in methane.
4. Draw the Lewis structures of the following. What shape are they? Are they polar molecules?
a) H2SO4 (sulfuric acid)
b) PH3
5. The following are ionic compounds. Draw the Lewis stuctures
a) calcium chloride, CaCl2, is an ionic halide that is solid at room temperture
b) K3PO4 is an ionic salt used as a food additive
6. Phosphorus, P, is an element that can form 5 covalent bonds.
a) draw a Lewis structure of the polyatomic ion PO4 (3-)
b) Lithium iron phosphate LiFePO4 is a compound used in batteries. Draw a possible Lewis structure of it.
7. Ethene is a nonpolar molecule. If you substitue two hydrogen atoms with two fluorine atoms you have difluoroethene. Is difluoroethene a polar molecule? Is there a possible difference in polarity between 1,1-difluoroethene and 1,2-difluoroethene?
8. What intermolecular forces might there be in a solution of HCl?
9. Which solid has a lower melting point in the following pairs:
a) sugar or salt?
b) SiO2 (silicon dioxide) or CaCl2 (calcium chloride)
c) Aluminium or quartz?
10. BH3, BeH2 and LiH are compounds that do not follow Lewis`s octet rule. Why?
11. What is the difference between quartz and glass?
Part 2
1. Fluorine is the most electronegative element. True or false?
2. Orbital theory can explain the structure of all known molecules. True or false?
3. If a molecule has 3 bond pairs and 1 lone pair of electrons, then the shape of the molecule will be tetrahedral. True or false?
4. Metals are found in group 15 of the periodic table. True or false?
5. Boron usually forms 3 covalent bonds. True or false?
6. In orbital theory a double bond involves one sigma bond and one pi bond. True or false?
7. Carbon can form sp, sp2 and sp3 hybrid orbitals. True or false?
8. Hybridized orbitals exist in non-bonded atoms. True or false?
9. A hybridized orbital can hold 3 or 4 electrons. True or false?
10. The sharing of electrons results in a covalent bond. True or false?
Fill in the blanks
11. A difference in electronegativity between atoms of 2.2 results in _______________ bond.
12. A molecule with an overall negative charge, such as NO3- is called a ____________________ ion.
13. An ion with a positive charge is called a ________________.
14. A molecule where the central atom forms 3 bonds, all on the same plane, has a shape called _____________________ planar.
15. Dipole-dipole forces are stronger than ______________________ forces.
Answers: 1.T, 2.F, 3.F, 4.F, 5.T, 6.T, 7.T, 8.F, 9.F, 10.T
11. ionic, 12. polyatomic, 13. cation, 14. trigonal, 15. London
------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Review Questions, Chemistry Test, Friday 3 Dec., 2010
There are also questions on page 145, 146, and 147 of the text that you can try.
1. maltose is a
a) amino acid
b) protein
c) sugar
d) nucleic acid
2. Glucose + Fructose is
a) a dipeptide
b) a disaccharide
c) a dinucleotide
d) a diol
3. A glycosidic bond occurs
a) between glucose and another glucose
b) between glucose and an amino acid
c) between glucose and a phosphate group
d) there is no such thing as a glycosidic bond
4. In a DNA helix, adenine pairs with
a) uracil
b) guanine
c) cytosine
d) thymine
5. One example of a sugar with 5 carbons is:
a) ribose
b) fructose
c) lactose
d) cellulose
6. True or False questions
a) The carbon - fluorine (C-F) bond is very strong. True or False?
b) Plexiglass is a plastic that looks a lot like glass. True or False?
c) PVC is another name for polyvinylcytosine. True or False?
d) Cross links between polymers can be made using inorganic elements such as sulfur (S). True or False?
e) Nylon is a natural polyamide polymer. True or False?
f) A vinyl group has 3 carbons in it. True or False?
g) Van der Waals forces are stronger than covalent bonds. True or False?
h) Glucose + Galactose makes the disaccharide mannose. True or False?
7. Using any 2 amino acids (page 119 of the text book), draw the structure of a dipeptide.
8. If a polymer is made from 2-methylpentene, what is the repeating unit in the polymer?
9. Draw the structure of a cyclic fructose molecule.
10. What are two differences between cellulose & starch.
11. The most common plastic is polyethylene. What is the monomer used to make this polymer?
12. Which of these does NOT have a hydroxyl group: glucose, ethanediol, cellulose, or 1,3-butadiene.
13. What is the structure of hydrogen peroxide?
14. How many polypeptide chains are there in hemoglobin (a blood protein)?
15. Name two aldoses (aldohexoses)
16. Name two kinds of cells in the body that have oligosaccharides on the surface.
Answers:
1. Maltose - if it ends in ose it is probably a sugar. Yes, maltose is a sugar
2. disaccharide
3. between a glucose and a glucose. A glycosidic bond is a bond between saccharides
4. T, or thymine
5. ribose is a 5 carbon sugar.
6. a) true b) true c) false PVC = polyvinylchlorine d) true e) false, nylon is a synthetic polyamide, f) false, vinyl has 2 carbons g) false, Van der Waals are weaker
h) false - mannose is a monosaccharide that you don't need to know the name of. beta-galactose + beta-glucose is lactose. NOTE lactose can also be beta-galactose + alpha-glucose.
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Review Questions for Organic Chemistry Quiz, 26 Nov. 2010
1. Draw the structural formula of the following:
a) s-butyl group
b) 3-iodopentan-2-one
c) 2-methylbutanal
d) acetone
e) 2-ethyl-3-methylbenzene
f) 4,4,-dimethylpent-1-yne
g) methoxyethane
2. True or false questions
a) Glycerol is an alcohol. True or false?
b) Hydration is an addition reaction. True or false?
c) Benzene + Chlorine --> Chlorobenzene is an example of an addition reaction. True or false?
d) Hexane has a lower boiling point than hexanol. True or false?
e) Sulfuric acid is a common catalyst in organic chemical reactions. True or false?
f) -COOH is a carboxyl group found in carboxylic acids. True or false?
g) Trichlorofluoromethane has only one H atom. True or false?
h) 2-methylhexane is a structural isomer of heptane. True or false?
i) 3-methylpentane-3-ol is a secondary alcohol. True or false?
j) 3,4-dimethynonane is an unsaturated hydrocarbon.
3. Which molecule is more soluable in water, butanol or butane? Why?
4. What are the products of a combustion reaction involving octane-2,3-diol?
5. What could be the name of a molecule that has the general formula R-OH?
(1) methanol;
(2) methane;
(3) methyl methanoate;
(4) methanoic acid.
6. What is the total number of carbons in a ethyl group?
7. Fill in the blanks.
a) The fermentation of sugar will produce carbon dioxide and ____________.
b) 1,3-butadiene has two __________________________ .
c) Saturated hydrocarbons can be separated by distillation because they have different __________________________ .
d) Methanol is _____________ hydrophilic than methane.
e) Hydration is the addition of _________________ to an alkene.
f) A _____________________is formed when a carboxylic acid reacts with an alcohol.
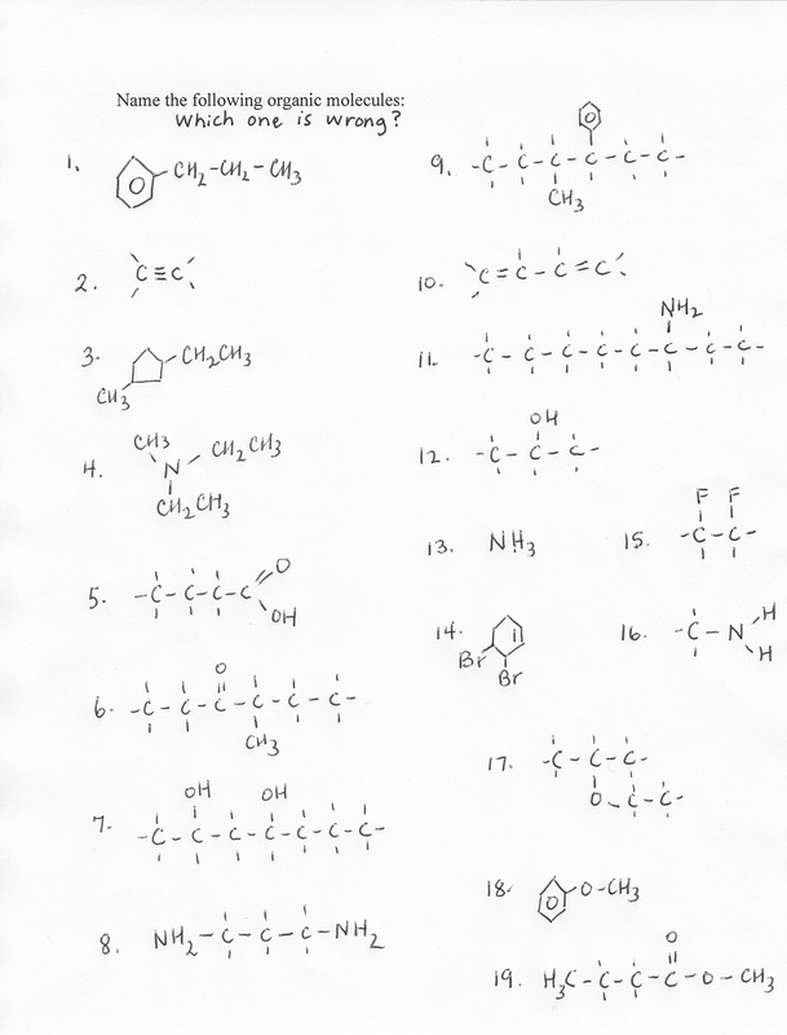
8. Name the structures in the picture. There are 19 of them. One of them is wrong.
These are the questions that were given in class on Wed. 26 Jan.
[Answers for questins 1 to 12 are given after question 12]
1. The equation representing the predominant reaction of sodium ethanoate, NaCH3COO, with water is
answer: d) CH3COO- + H20 <--> CH3COOH + OH-
2. What is the pH of the solution of when a small amount of 0.040 mol NaOH(s) is added to
1.00 L of 0.050 M HCl?
a) 2.00 b) 1.40 c) 1.30 d) 7.00 e) 8.50
3. Which of the following represents a redox reaction?
answer: a) SiCl4 + 2Mg --> Si + 2MgCl2
4. In a nickel-cadmium barttery: NiO2 (s) + Cd(s) + 2H2) --> Ni(OH)2 + Cd(OH)2 . Which of the following occurs at the anode as the reaction proceeds?
a) NiO2 gains 2e- and forms Ni(OH)2(s)
b) NiO2 loses 2e- and forms Ni(OH)2(s)
C) Cd gains 2e- and forms Cd(OH)2(s)
d) Cd loses 2e- and forms Cd(OH)2(s)
5. Consider the following reaction:
2NO(g)+ O₂(g) à NO₂ (g) ∆H = -114 kJ
How could the rate of this reaction be increased?
A. Reduce the pressure.
B. Increase the volume.
C. Remove some NO2(g) .
D. Increase the temperature.
6. (picture can not copy here ). The answer is d.
7. An activated complex can be described as
A. a particle of maximum KE and minimum PE.
B. a stable particle found in a reaction mechanism.
C. an unstable particle that is neither reactant nor product.
D. a particle which is first used then regenerated in a reaction mechanism.
8. Which of the following could result in an increase in reaction rate?
A. an increase in the activation energy
B. an increase in the reaction enthalpy
C. an increase in the frequency of collisions
D. an increase in the potential energy of the activated complex
9. Consider the following equilibrium: CO₂(g) + 2 H₂(g) à CH₃OH(g) ∆H = - 91 kJ +
Which of the factors below would increase the concentration of CH₃OH at equilibrium?
A. an addition of CO₂
B. an increase in the volume
C. a decrease in the pressure
D. an increase in the temperature
10. What does a buffer solution usually contain?
A. a strong acid and its conjugate acid
B. a strong acid and its conjugate base
C. a weak acid and its conjugate acid
D. a weak acid and its conjugate base
11. Acidified potassium permanganate (KMnO4) solution is often used in redox
titrations. Permanganate reacts with Sn2+ as follows: MnO₄¯ + Sn²⁺ --> Mn²⁺ + Sn⁴⁺
When the equation is balanced, the number of Sn²⁺ ions is:
a) 1 b) 2 c) 3 d) 4 e) 5
12. What is the reducing agent in the reaction in question 11?
a) Mn²⁺ b) Sn⁴⁺ c) Sn²⁺ d) MnO₄⁻
Answers for 1 - 12:
1. d, 2. a, 3. a, 4. d, 5. d, 6. d,
7. c, 8. c, 9. a, 10. d, 11. e, 12. c
13. Balance this redox reaction H₂S + CrO₄²⁻ --> S₈ + Cr³⁺ in acidic solution
80 H+ + 24 H2S + 16 (CrO4)2- --> 3 S8 +16 Cr3+ + 64 H2O
14. Hydrogen peroxide decomposes according to the following thermochemical reaction:
H2O2(l) → H2O(l) + 1/2 O2(g); ΔH = -98.2 kJ Molar mass of H2O2 is 34.0
Calculate the change in enthalpy, ΔH, when 1.00 g of hydrogen peroxide decomposes.
-2.89 kJ
15. How much energy in kJ must be removed from 225 g of water in order to lower its temperature from 25.0 C to 10.0 C? 14.1 kJ
16. When 13.4g of ammonium chloride is mixed with 2.00x102 g of water it dissociates into ions and causes the temperature of the solution to drop from 20.0oC to 15.3oC. Determine the molar enthalpy of solution for ammonium chloride. (Ans: +15.7kJ/mol)
17. The enthalpy of combustion for H2, C(graphite) and CH4 given below. Calculate the standard enthalpy of formation ∆Hf for CH4.
(1) H2(g) + 0.5 O2(g) -> H2O(l) ∆H°f = -285.8 kJ/mol
(2) C(graphite) + O2(g) -> CO2(g) ∆H°f = -293.5 kJ/mol
(3) CH4(g) + 2O2(g) -> CO2(g) + 2H2O(l) -890.4 kJ/mol
18 . From the following data,
CH4 + 2O2 -> CO2 + 2H2O ∆H = -890 kJ/mol
H2O(l) -> H2O(g) ∆H = 44 kJ/mol at 298 K
Calculate the enthalpy of the reaction
CH4 + 2 O2(g) -> CO2(g) + 2 H2O(g) ∆H = ? - 802 kJ/mol
19. The enthalpies of formation of H2O, H2S, and SO2 are -285.8, -20.6 and -296.8 kJ/mol respectively. Calculate the standard enthalpy of change for the reaction, 2 H2S + SO2 -> 3 S + 2 H2O
All reactants and products are at their standard states.
-233.6 kJ (Use Hess's Law)
20. What is the enthalpy change when 12.8g H2(g) reacts with excess Cl2(g) to form HCl(g)? H2(g) + Cl2(g) à 2HCl(g) ∆H = -184.6kJ -1.17 X 10 to the 3 kJ
21. A burner on an electric stove has a heat capacity of 345J/K. What is the value of q, in kilojoules, as the burner cools from a temperature of 467°C to a room temperature of 23°C? -153 kJ
22. What mass of water, in kilograms, can be heated from 5.5°C to 55.0°C by 9.09 x 10 ⁱ⁰ J of heat? 4.39 X 10 to the 5 kg
23. A 454g block of lead is at an initial temperature of 22.5°C. What will be the temperature of the lead after it absorbs 4.22kJ of heat from its surroundings? 95.1 C
24. Calculate the value of the equilibrium constant, Kc , for the system shown, if 0.1908 moles of CO2, 0.0908 moles of H2, 0.0092 moles of CO, and 0.0092 moles of H2O vapor were present in a 2.00 L reaction vessel were present at equilibrium
Co2 + H2 <--> CO + H2O 4.9 x 10 to the -3 = K
25. Initially, a mixture of 0.100 M NO, 0.050 M H2, 0.100 M H2 O was allowed to reach equilibrium (initially there was no N2 ). At equilibrium the concentration of NO was found to be 0.062 M. Determine the value of the equilibrium constant, Kc , for the reaction: 2 NO + 2H2 <--> N2 + 2 H2O K = 650
26. A container is charged with 3.00 atm of dinitrogen tetroxide gas and 2.00 atm of nitrogen dioxide gas at 25oC and allowed to reach equilibrium. It was found that the pressure of the nitrogen dioxide decreased by 0.952 atm. Estimate the value of Kp for this system: Kp is similar to Kc and the calculations are done the same way except that you use pressure in atmospheres instead of concentration. N2O4 <--> 2 NO2
K = 0.3160
27. Phosgene (COCl2) is a poisonous gas that dissociates at high temperature into two other poisonous gases, carbon monoxide and chlorine. The equilibrium constant Kp = 0.0041 at 600°K. Find the equilibrium composition of the system after 0.124 atm of COCl2 is allowed to reach equilibrium at this temperature. Assume that x is very small.
0.104 atm for COCl2 and 0.0206 atm for CO and Cl2
28. Calculate the concentrations for each species present in a 0.1000 M aqueous solution of nitrous acid (Ka = 6.0 x 10-4). HNO2(aq) + H2O(l) H3O+(aq) + NO2-(aq)
[HNO2] = 0.100-x = 0.0926M
29. What is the hydronium ion concentration in a water solution that is 0.050 M NaOH?
2 x 10 -13 M
30. What is the hydroxide ion concentration in a water solution that is 4.0 x 10-5 M H3O+?
2.5 x 10 -10 M
31. What is the pKa of acetic acid, if Ka for acetic acid is 1.78 x 10-5?
4.75
32. Calculate the value of the ionization constant for the ammonium ion, Ka, if the pKa is 9.74.
1.82 x 10 -10
33. What is the pKb for methyl amine, if the value of Kb for methyl amine is 4.4 x 10-4?
3.36
34. Calculate the value of the ionization constant, Kb, for aniline if the pKb is 9.38.
4.17 x 10 -10
35. The Ka for acetic acid is 1.7 x 10-5. What is the value of Kb for the acetate ion?
5.9 x 10 -10
36. The Kb for aniline is 3.8 x 10-10. What is the value of Ka for the anilonium ion?
2.6 x 10 -5
37. What is the pH of a 0.400 M KBr solution?
neutral, pH = 7
38. To determine the initial concentration of a reactant A, we need to know: e
a) The final concentration of A
b) The length of time, t, the reaction ran to reach the final concentration.
c) The order of the reaction or enough information to determine it.
d) The rate constant, k, for the reaction or enough information to determine it.
e) All of the above.
39. Calculate the solubility product constant (Ksp) for lead(II) chloride, if 50.0 mL of a saturated solution of lead(II) chloride was found to contain 0.2207 g of lead(II) chloride dissolved in it. PbCl2(s) --> Pb2+(aq) + 2 Cl-(aq)
1.61 x 10 -5
40. Estimate the solubility of Ag2CrO4 in pure water if the solubility product constant for silver chromate is 1.1 x 10-12.
Ag2CrO4(s) --> 2 Ag+(aq) + CrO42-(aq)
[CrO4] = 6.5 x 10 -5 M
41. Estimate the solubility of barium sulfate in a 0.020 M sodium sulfate solution. The solubility product constant for barium sulfate is 1.1 x 10-10. BaSO4(s) --> Ba2+(aq) + SO42-(aq) [note: common ions!]
5.5 x 10 -9 M is x in the ICE table
42. 25.0 mL of 0.0020 M potassium chromate are mixed with 75.0 mL of 0.000125 M lead(II) nitrate. Will a precipitate of lead(II) chromate form. Ksp of lead(II) chromate is 1.8 x 10-14. yes we will look at this in class tomorrow (Thursday)
Review Questions, Redox Quiz Wed. 26 Jan 2010
see homework questions from the past few days as well
1. In a redox reaction, a reducing agent becomes oxidized. True or False?
2. An atom or ion is oxidized if it's oxidation number increases in a redox reaction. True or false?
3. Oxidation occurs in the anode half cell in an electrochemical cell. True or false?
4. An electrochemical cell has two solid electrodes. True or false?
5. If an ion loses electrons in a redox reaction, it has been oxidized. True or false?
6. Balance this redox reaction: H₂S + CrO₄²⁻ --> S₈ + Cr³⁺ in acidic solution.
7. In the reaction in question 6, what is the reducing agent?
8. Write a chemical equation showing the oxidation of an iron ion.
9. What is the purpose of the salt bridge in a galvanic cell?
10. Describe what happens to electrons in an electrochemical cell. Draw pictures if needed.
--------------------------------------------------------------
Review Questions, Chemistry Quiz, Thursday 20 Jan., 2010
I'll be adding questions until about 8:30 p.m. this evening (19 Jan)
1. Strong acids conduct electricity ____________________ than weak acids.
2. What is the definition of a Lewis acid?
3. Naturally occurring acids are _____________ acids (strong or weak?)
4. If you have a 1.2 M solution of NaOH, what is the [OH-]?
5. What is the difference between a monoprotic acid and a diprotic acid?
6. Name 2 strong acids.
7. What is a neutralization reaction?
8. What is a hydronium ion?
9. F- is a weak base. What is its conjugate acid?
10. HClO4 is a strong acid. What is its conjugate base? Is its conjugate base a weak base or a strong base?
11. If the Ka of an acid HA is 3 x 10-5, what is the Kb of the ion A-? What is the pKa of HA?
12. In the reaction, H2PO4- + HS- --> H2S + HPO42- which ions are the acid and the base?
13. In the reaction B + H20 --> BH + OH- where B is a base, what is the conjugate acid of B?
14. What is the difference between a cation and an anion?
15. A strong acid reacts with a weak base and a salt is formed. Will it probably be an acidic salt or a basic salt?
16. What does the word alkaline mean?
17. Write a balanced chemical equation for the reaction of Ba(OH)2 in water.
18. The metal oxide, MgO (solid) is put in a beaker of water. A small amount of MgO dissolves in the water. Is the aqueous solution acidic or basic?
19. What is Tris?
20. Why is HCO3- called amphoteric?
21. What are 3 differences between electrophiles and nucleophiles?
22. What is a coordinate covalent bond?
23. If a a substance R-COOH has a pKa of 5 and it is in a solution of pH 7, is that substance likely to be charged or neutral?
24. Acid A has a pKa of 1 and acid B has a pKa of 4. Which acid is stronger?
25. If HA has a pH of 4.5, what is its pOH?
26. Buffers are often made with a weak base and its ___________________ .
27. Write the chemical equation for any hydrolysis reaction.
28. Write the chemical equation for any neutralization reaction.
29, If a solution 0.5 M of acid, HA, is 25% ionized at equilibrium, what is the [HA] at equilibrium?
30. What is the pH of a 0.15 M solution of methylamine CH3NH2 if the Kb = 4.4 x 10-4 ?
31. What is the conjugate acid of CH3NH2?
32. What is the Kw and pKw of water?
33. If you wanted to make a 1L solution of pH = 2, how much HCL should you add to 1L of water? Ignore the volume change after adding HCl.
34. pH is dependent on concentration, true or false?
35. What is an electrolyte?
Part B
1. Which of the following 0.1 M solutions will have the greatest electrical conductivity?
a) HNO2
b) H2SO3
c) H3PO4
d) C6H5OH
2. Consider the following equilibrium
2H2O(l ) ⇄ H3O+ (aq) + OH- (aq)
A small amount of HCl is added to water and equilibrium is reestablished. When comparing the new equilibrium with the original equilibrium,
a) [H3O+] and pH both decreased
b) [H3O+] and pH both increased
c) [H3O+] increased and pH decreased
d) [H3O+] decreased and pH increased
3. A Brönsted-Lowry base is defined as a chemical species that
a) accepts protons
b) neutralizes acids
c) donates electrons
d) produces hydroxide ions in solution
4. In a solution with a [OH-] of 1.5 x 10-4 M, the [H3O+] is
a) 6.7 x 10-11 M
b) 1.0 x 10-7 M
c) 1.5 x 10-4 M
d) 1.2 x 10-2 M
5. The pH of pure water is 6.52 at 60°C. The [OH-] is
a) 3.3 x 10-8 M
b) 1.0 x 10-7 M
c) 3.0 x 10-7 M
d) 8.1 x 10-1 M
6. Which of the following oxides, when dissolved in water, will produce the most basic solution?
a) BaO
b) CO2
c) SO2
d) ClO
7. Which of the following could act as a Brönsted-Lowry acid, but not as a Brönsted-Lowry base?
a) HC1O4(aq)
b) H2O(l)
c) NH3(aq)
d) HCO3(aq)
8. The strength of an acid depends upon its
a) E°
b) pH
c) concentration
d) degree of ionization
9. Consider the following equilibrium:
HX- + HZ- ⇄ H2X + Z 2-
The reactants are favoured. The strongest acid is
a) Z 2-
b) HZ
c) HX
d) H2X
10. The pH of a 0.025 M NaOH solution is
a) 0.94
b) 1.60
c) 12.40
d) 13.06
Part C
1. Nicotinic acid, HC6H4NO2, is a weak acid found in vitamin B.
Calculate the pH of 0.100 M HC6H4NO2 (Ka = 1.4 x 10-5).
2. What is the [H3O+] in a solution formed by adding 60.0 mL of water to 40.0 mL of 0.040 M KOH?
3. Calculate the pH in 100.0 mL of 0.400 M CH3COOH (Ka = 1.8 x 10-5 is a weak acid)
4. The salt NaCN dissolves in water and forms a slightly basic solution. Write the dissociation equation for
NaCN in water. (the chemical equations for what happens when NaCN dissolves in water)
--------------------------------------------------------------------------------------------------------------
for Tuesday, 14 Dec
Don`t forget to review WHMIS symbols for hazardous materials! Page 790 & 791
Review questions, pages 282 and 283 of text
Self-quiz, pages 286 - 289 of the text
Part 1
1. Draw an example of a metallic bonding.
2. Draw an example of an ionic crystal.
3. Draw the shape and arrangement of the sp3 hybrid orbitals in methane.
4. Draw the Lewis structures of the following. What shape are they? Are they polar molecules?
a) H2SO4 (sulfuric acid)
b) PH3
5. The following are ionic compounds. Draw the Lewis stuctures
a) calcium chloride, CaCl2, is an ionic halide that is solid at room temperture
b) K3PO4 is an ionic salt used as a food additive
6. Phosphorus, P, is an element that can form 5 covalent bonds.
a) draw a Lewis structure of the polyatomic ion PO4 (3-)
b) Lithium iron phosphate LiFePO4 is a compound used in batteries. Draw a possible Lewis structure of it.
7. Ethene is a nonpolar molecule. If you substitue two hydrogen atoms with two fluorine atoms you have difluoroethene. Is difluoroethene a polar molecule? Is there a possible difference in polarity between 1,1-difluoroethene and 1,2-difluoroethene?
8. What intermolecular forces might there be in a solution of HCl?
9. Which solid has a lower melting point in the following pairs:
a) sugar or salt?
b) SiO2 (silicon dioxide) or CaCl2 (calcium chloride)
c) Aluminium or quartz?
10. BH3, BeH2 and LiH are compounds that do not follow Lewis`s octet rule. Why?
11. What is the difference between quartz and glass?
Part 2
1. Fluorine is the most electronegative element. True or false?
2. Orbital theory can explain the structure of all known molecules. True or false?
3. If a molecule has 3 bond pairs and 1 lone pair of electrons, then the shape of the molecule will be tetrahedral. True or false?
4. Metals are found in group 15 of the periodic table. True or false?
5. Boron usually forms 3 covalent bonds. True or false?
6. In orbital theory a double bond involves one sigma bond and one pi bond. True or false?
7. Carbon can form sp, sp2 and sp3 hybrid orbitals. True or false?
8. Hybridized orbitals exist in non-bonded atoms. True or false?
9. A hybridized orbital can hold 3 or 4 electrons. True or false?
10. The sharing of electrons results in a covalent bond. True or false?
Fill in the blanks
11. A difference in electronegativity between atoms of 2.2 results in _______________ bond.
12. A molecule with an overall negative charge, such as NO3- is called a ____________________ ion.
13. An ion with a positive charge is called a ________________.
14. A molecule where the central atom forms 3 bonds, all on the same plane, has a shape called _____________________ planar.
15. Dipole-dipole forces are stronger than ______________________ forces.
Answers: 1.T, 2.F, 3.F, 4.F, 5.T, 6.T, 7.T, 8.F, 9.F, 10.T
11. ionic, 12. polyatomic, 13. cation, 14. trigonal, 15. London
------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Review Questions, Chemistry Test, Friday 3 Dec., 2010
There are also questions on page 145, 146, and 147 of the text that you can try.
1. maltose is a
a) amino acid
b) protein
c) sugar
d) nucleic acid
2. Glucose + Fructose is
a) a dipeptide
b) a disaccharide
c) a dinucleotide
d) a diol
3. A glycosidic bond occurs
a) between glucose and another glucose
b) between glucose and an amino acid
c) between glucose and a phosphate group
d) there is no such thing as a glycosidic bond
4. In a DNA helix, adenine pairs with
a) uracil
b) guanine
c) cytosine
d) thymine
5. One example of a sugar with 5 carbons is:
a) ribose
b) fructose
c) lactose
d) cellulose
6. True or False questions
a) The carbon - fluorine (C-F) bond is very strong. True or False?
b) Plexiglass is a plastic that looks a lot like glass. True or False?
c) PVC is another name for polyvinylcytosine. True or False?
d) Cross links between polymers can be made using inorganic elements such as sulfur (S). True or False?
e) Nylon is a natural polyamide polymer. True or False?
f) A vinyl group has 3 carbons in it. True or False?
g) Van der Waals forces are stronger than covalent bonds. True or False?
h) Glucose + Galactose makes the disaccharide mannose. True or False?
7. Using any 2 amino acids (page 119 of the text book), draw the structure of a dipeptide.
8. If a polymer is made from 2-methylpentene, what is the repeating unit in the polymer?
9. Draw the structure of a cyclic fructose molecule.
10. What are two differences between cellulose & starch.
11. The most common plastic is polyethylene. What is the monomer used to make this polymer?
12. Which of these does NOT have a hydroxyl group: glucose, ethanediol, cellulose, or 1,3-butadiene.
13. What is the structure of hydrogen peroxide?
14. How many polypeptide chains are there in hemoglobin (a blood protein)?
15. Name two aldoses (aldohexoses)
16. Name two kinds of cells in the body that have oligosaccharides on the surface.
Answers:
1. Maltose - if it ends in ose it is probably a sugar. Yes, maltose is a sugar
2. disaccharide
3. between a glucose and a glucose. A glycosidic bond is a bond between saccharides
4. T, or thymine
5. ribose is a 5 carbon sugar.
6. a) true b) true c) false PVC = polyvinylchlorine d) true e) false, nylon is a synthetic polyamide, f) false, vinyl has 2 carbons g) false, Van der Waals are weaker
h) false - mannose is a monosaccharide that you don't need to know the name of. beta-galactose + beta-glucose is lactose. NOTE lactose can also be beta-galactose + alpha-glucose.
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Review Questions for Organic Chemistry Quiz, 26 Nov. 2010
1. Draw the structural formula of the following:
a) s-butyl group
b) 3-iodopentan-2-one
c) 2-methylbutanal
d) acetone
e) 2-ethyl-3-methylbenzene
f) 4,4,-dimethylpent-1-yne
g) methoxyethane
2. True or false questions
a) Glycerol is an alcohol. True or false?
b) Hydration is an addition reaction. True or false?
c) Benzene + Chlorine --> Chlorobenzene is an example of an addition reaction. True or false?
d) Hexane has a lower boiling point than hexanol. True or false?
e) Sulfuric acid is a common catalyst in organic chemical reactions. True or false?
f) -COOH is a carboxyl group found in carboxylic acids. True or false?
g) Trichlorofluoromethane has only one H atom. True or false?
h) 2-methylhexane is a structural isomer of heptane. True or false?
i) 3-methylpentane-3-ol is a secondary alcohol. True or false?
j) 3,4-dimethynonane is an unsaturated hydrocarbon.
3. Which molecule is more soluable in water, butanol or butane? Why?
4. What are the products of a combustion reaction involving octane-2,3-diol?
5. What could be the name of a molecule that has the general formula R-OH?
(1) methanol;
(2) methane;
(3) methyl methanoate;
(4) methanoic acid.
6. What is the total number of carbons in a ethyl group?
7. Fill in the blanks.
a) The fermentation of sugar will produce carbon dioxide and ____________.
b) 1,3-butadiene has two __________________________ .
c) Saturated hydrocarbons can be separated by distillation because they have different __________________________ .
d) Methanol is _____________ hydrophilic than methane.
e) Hydration is the addition of _________________ to an alkene.
f) A _____________________is formed when a carboxylic acid reacts with an alcohol.
8. Name the structures in the picture. There are 19 of them. One of them is wrong.